Introduction
AI4Solvents is a free, accessible web server designed to predict various properties of solvents. Currently, it includes 25 properties for molecular solvents, 12 properties for ionic liquids, 4 properties for deep eutectic solvents, and 7 properties for refrigerants, with a total of 51,794, 86,566, 9,955, and 2,573 data points, respectively. In the future, we will continuously expand the range of solvent types, incorporating more properties and data to enhance the platform's capabilities.
Dataset description
|
Solvent type
|
Property
|
Unit
|
Data points
|
| Molecular solvents |
Auto ignition temperature |
K |
571 |
| Molecular solvents |
Bioconcentration factor |
1 |
590 |
| Molecular solvents |
Hansen solubility parameter dispersion δD. |
MPa⁰·⁵ |
1038 |
| Molecular solvents |
Hansen solubility parameter H2 |
MPa⁰·⁵ |
1017 |
| Molecular solvents |
Hansen solubility parameter Polar |
MPa⁰·⁵ |
1018 |
| Molecular solvents |
Flash point |
K |
513 |
| Molecular solvents |
Standard Gibbs energy of formation |
kJ/mol |
757 |
| Molecular solvents |
Standard enthalpy of formation |
kJ/mol |
1060 |
| Molecular solvents |
Normal enthalpy of fusion |
kJ/mol |
750 |
| Molecular solvents |
Hildebrandt solubility parameter at 298 K |
MPa⁰·⁵ |
1385 |
| Molecular solvents |
Enthalpy of vaporization at 298 K |
kJ/mol |
426 |
| Molecular solvents |
Fathead Minnow 96-H LC50 |
mol/L |
706 |
| Molecular solvents |
Toxicity (oral rat) |
mol/kg |
4905 |
| Molecular solvents |
Liquid molar volume at 298 K |
cc/mol |
1057 |
| Molecular solvents |
Octanol-water partition coefficient |
1 |
12194 |
| Molecular solvents |
Aqueous solubility |
mol/L |
2566 |
| Molecular solvents |
Acentric factor |
1 |
1724 |
| Molecular solvents |
Permissible exposure limit |
mol/m³ |
423 |
| Molecular solvents |
Critical pressure |
bar |
775 |
| Molecular solvents |
Photochemical oxidation potential |
1 |
607 |
| Molecular solvents |
Acid dissociation constant |
1 |
1634 |
| Molecular solvents |
Normal boiling point |
K |
5277 |
| Molecular solvents |
Critical temperature |
K |
777 |
| Molecular solvents |
Normal melting point |
K |
9250 |
| Molecular solvents |
Critical volume |
cc/mol |
774 |
| Ionic liquids |
Melting point |
K |
2673 |
| Ionic liquids |
Glass transition temperature |
K |
798 |
| Ionic liquids |
Thermal decomposition temperature |
K |
2780 |
| Ionic liquids |
Cytotoxicity towards the leukemia rat cell line IPC-81 log10EC50 |
1 |
355 |
| Ionic liquids |
Electrical conductivity |
ln(S/m) |
2168 |
| Ionic liquids |
Viscosity |
ln(mPa·s) |
15368 |
| Ionic liquids |
Surface tension |
mN/m |
6051 |
| Ionic liquids |
Refractive index |
1 |
2963 |
| Ionic liquids |
Heat capacity |
ln(J mol K⁻¹) |
11521 |
| Ionic liquids |
Thermal conductivity |
W m⁻¹ K⁻¹ |
606 |
| Ionic liquids |
Density |
kg/m³ |
31167 |
| Ionic liquids |
CO₂ solubility |
mol CO₂/mol IL |
10116 |
| Deep-eutectic Solvents |
Melting point |
K |
3701 |
| Deep-eutectic Solvents |
Density |
g/cm³ |
2384 |
| Deep-eutectic Solvents |
Viscosity |
ln(mPa·s) |
1640 |
| Deep-eutectic Solvents |
CO₂ solubility |
mol CO₂/mol DES |
2230 |
| Refrigerants |
Normal boiling point |
K |
1016 |
| Refrigerants |
Critical temperature |
K |
336 |
| Refrigerants |
Critical pressure |
bar |
321 |
| Refrigerants |
Heat of vaporization at 298K |
kJ/mol |
158 |
| Refrigerants |
Acentric factor |
1 |
227 |
| Refrigerants |
Global warming potential |
1 |
279 |
| Refrigerants |
Ozone depletion potential |
1 |
236 |
Modeling framework
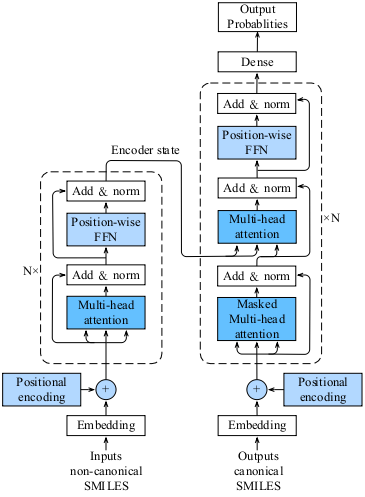
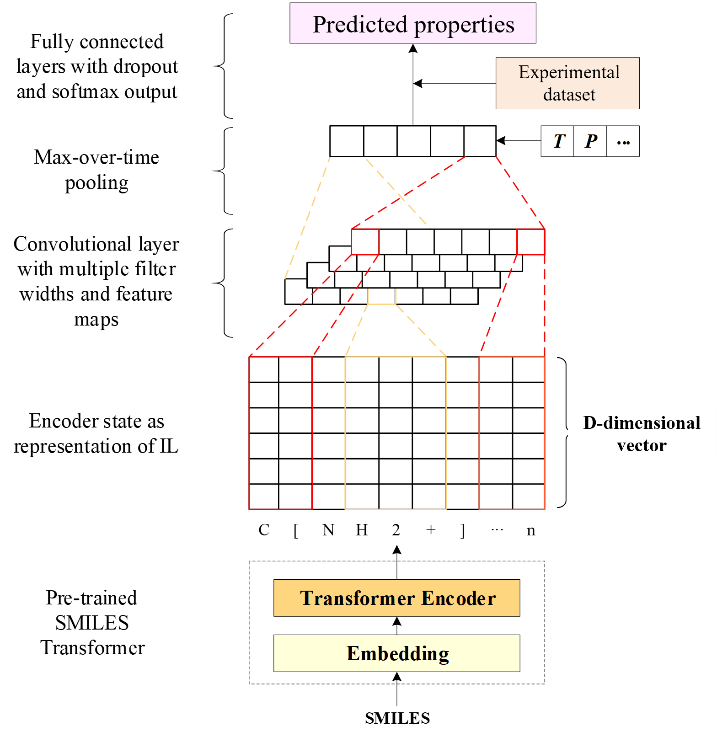
This work is based on the Transformer-CNN model to predict the properties of solvents, whose structures are respectively shown in Figures A and B below.
Model performance
|
Solvent type
|
Property
|
MAE
|
RMSE
|
R²
|
| Ionic liquids |
Melting point |
25.86±0.20 |
34.92±0.30 |
0.783±0.004 |
| Ionic liquids |
Glass transition temperature |
9.88±0.23 |
15.96±0.23 |
0.708±0.009 |
| Ionic liquids |
Thermal decomposition temperature |
21.88±0.20 |
32.63±0.32 |
0.816±0.004 |
| Ionic liquids |
Cytotoxicity towards the leukemia rat cell line IPC-81 log₁₀EC₅₀ |
0.2007±0.0039 |
0.2777±0.0026 |
0.9400±0.0011 |
| Ionic liquids |
Electrical conductivity |
0.350±0.010 |
0.530±0.012 |
0.888±0.005 |
| Ionic liquids |
Viscosity |
0.326±0.002 |
0.555±0.006 |
0.883±0.002 |
| Ionic liquids |
Surface tension |
2.34±0.15 |
3.65±0.24 |
0.835±0.021 |
| Ionic liquids |
Refractive index |
0.0055±0.0001 |
0.0086±0.0002 |
0.954±0.002 |
| Ionic liquids |
Heat capacity |
0.0021±0.0001 |
0.0029±0.0001 |
0.988±0.001 |
| Ionic liquids |
Thermal conductivity |
13.24±0.26 |
26.20±0.43 |
0.979±0.001 |
| Ionic liquids |
Density |
15.89±3.18 |
24.30±3.38 |
0.990±0.003 |
| Ionic liquids |
CO₂ solubility |
0.0343±0.0004 |
0.0595±0.0014 |
0.937±0.003 |
| Molecular solvents |
Auto ignition temperature |
55.787±9.133 |
82.339±16.366 |
0.590±0.102 |
| Molecular solvents |
Bioconcentration factor |
0.513±0.054 |
0.692±0.068 |
0.733±0.077 |
| Molecular solvents |
Hansen solubility parameter - dispersion δD |
0.545±0.038 |
0.735±0.080 |
0.828±0.027 |
| Molecular solvents |
Hansen solubility parameter - H2 |
1.403±0.209 |
2.113±0.502 |
0.792±0.080 |
| Molecular solvents |
Hansen solubility parameter - Polar |
1.776±0.164 |
2.419±0.236 |
0.614±0.118 |
| Molecular solvents |
Flash point |
11.454±1.634 |
15.971±3.230 |
0.936±0.024 |
| Molecular solvents |
Standard Gibbs energy of formation |
15.348±2.753 |
26.275±5.151 |
0.983±0.010 |
| Molecular solvents |
Standard enthalpy of formation |
18.542±3.912 |
33.365±10.303 |
0.980±0.009 |
| Molecular solvents |
Normal enthalpy of fusion |
3.358±0.789 |
5.530±3.028 |
0.798±0.112 |
| Molecular solvents |
Hildebrandt solubility parameter at 298 K |
0.967±0.126 |
1.575±0.228 |
0.837±0.045 |
| Molecular solvents |
Enthalpy of vaporization at 298 K |
3.174±0.801 |
5.069±1.372 |
0.821±0.116 |
| Molecular solvents |
Fathead Minnow 96-H LC50 |
0.763±0.220 |
1.002±0.266 |
0.516±0.240 |
| Molecular solvents |
Toxicity (oral rat) |
0.357±0.014 |
0.459±0.020 |
0.626±0.022 |
| Molecular solvents |
Liquid molar volume at 298 K |
0.006±0.002 |
0.015±0.012 |
0.947±0.085 |
| Molecular solvents |
Octanol-water partition coefficient |
0.286±0.008 |
0.417±0.013 |
0.946±0.004 |
| Molecular solvents |
Aqueous solubility |
0.522±0.044 |
0.749±0.083 |
0.872±0.032 |
| Molecular solvents |
Acentric factor |
0.067±0.008 |
0.119±0.028 |
0.840±0.081 |
| Molecular solvents |
Permissible exposure limit |
0.623±0.089 |
0.957±0.189 |
0.595±0.078 |
| Molecular solvents |
Critical pressure |
1.767±0.310 |
3.202±1.240 |
0.906±0.098 |
| Molecular solvents |
Photochemical oxidation potential |
0.134±0.019 |
0.218±0.034 |
0.825±0.064 |
| Molecular solvents |
Acid dissociation constant |
1.291±0.196 |
2.014±0.328 |
0.666±0.104 |
| Molecular solvents |
Normal boiling point |
14.549±1.395 |
33.094±17.190 |
0.859±0.108 |
| Molecular solvents |
Critical temperature |
15.975±2.189 |
26.379±6.925 |
0.936±0.030 |
| Molecular solvents |
Normal melting point |
31.334±0.900 |
45.857±2.030 |
0.806±0.016 |
| Molecular solvents |
Critical volume |
15.808±2.659 |
24.753±7.522 |
0.985±0.005 |
| Deep eutectic solvents |
Melting point |
19.7 |
28.23 |
0.8718 |
| Deep eutectic solvents |
Density |
0.0272 |
0.0414 |
0.9119 |
| Deep eutectic solvents |
Viscosity |
0.1015 |
0.1446 |
0.9934 |
| Deep eutectic solvents |
CO₂ solubility |
0.2584 |
0.3668 |
0.9275 |
| Refrigerants |
Normal boiling point |
2.058 |
3.747 |
0.859 |
| Refrigerants |
Critical temperature |
8.084 |
13.586 |
0.936 |
| Refrigerants |
Critical pressure |
7.017 |
10.817 |
0.981 |
| Refrigerants |
Heat of vaporization at 298 K |
0.633 |
1.275 |
0.969 |
Reference
-
[1]Accurate prediction of performance-related properties of refrigerants with machine learning and new small molecule descriptors[J].
Comput Chem Eng. 2025, 109264.
Cao P, Geng Y, Feng N, et al
-
[2]Large chemical language models for property prediction and high-throughput screening of ionic liquids[J].
Digital Discovery. 2025, 4(6): 1505-1517.
Qiu Y, Song Z, Chen G, et al.
-
[3]FusNet: unlocking molecular fusion properties through machine learning[J].
Front. Chem. Sci. Eng., 2025, 19, 81.
Chen J., Qiu Y., Song Z. et al.